One of Michael Snyder’s smartwatches is on the blink, but the Stanford University professor, sometimes described as “medicine’s most measured man”, remains an unmatched source of real-time health data. That data is not just from the Fitbit and two other smartwatches he flashes to the camera during our Zoom call but also from a smart ring, a glucose monitor on the back of his arm and two hearing aids that monitor his body temperature, heart rate, blood oxygen levels and blood pressure.
“Many hearing aids can now tell if you’ve fallen over and we’re seeing different sensors to monitor physiology,” says Snyder (pictured below, centre), who, as chair and director of genetics and personalised medicine at Stanford, is often asked to try new gadgets emerging from the booming market in wearable health-tech, predicted to be worth $380 billion (£300 billion) by 2028, up from $115 billion in 2021. The value of the “hearables” market alone will hit $146 billion by 2030, a report by GlobalData predicted last year.

For many years, Snyder was an eccentric outlier in this rigorous self-tracking of fitness and bodily functions, but the rest of the world is catching up. Gymgoers routinely check their mobile phone mid-workout to see if their heart rates, measured by a smartwatch, are hitting the requisite intensity levels. Some 15 per cent of Americans use sleep tracking apps, while glucose monitoring systems offered by the likes of ZOE, SuperSapiens and January AI – the last co-founded by Snyder – have gained huge followings thanks to their gamification of eating, pushing users to avoid spikes in blood sugar by eating healthily.
Despite criticism that these innovations cater largely to the “worried well” – the affluent and health-savvy middle class who can afford a £400 iWatch or £30-a-month ZOE subscription – Snyder views as overwhelmingly positive the emerging ability of individuals to monitor their own health data.
“Every car has a dashboard monitoring fuel, oil, water and lights, and you wouldn’t drive anywhere without looking at these things,” he says. “Why wouldn’t you want to look at information for your own body? It’s true that the people who can afford it are [doing it] first – that’s the reality with healthcare in most countries. Prices are high because companies need to recover their R&D costs. But prices will come down and, as we prove their utility, devices will become dirt cheap.”

Given the arrival of wearable tech, fitness apps and the mainstreaming of telemedicine during the pandemic, it is little surprise that research into digital health has also grown massively. But the scale of growth in digital health research is still remarkable. According to an analysis by Clarivate that is being presented at Times Higher Education’s Digital Health Summit at Stanford on 28 and 29 February, publications on these topics – which include everything from wearable devices and mobile apps to AI analytics, telemedicine and 3D printing of drugs – have risen nearly 70-fold between 2013 and 2022, from a mere 39 Web of Science-indexed papers to 2,641.
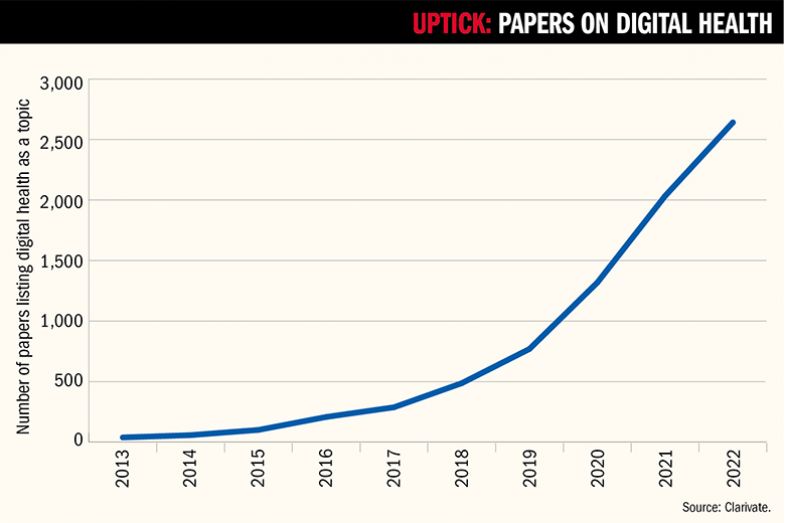
With investors sniffing round universities for the next big thing – they piled £21 billion into digital health start-ups in 2022 and £31 billion in 2021 – the golden age of digital health research could also be a very lucrative one for scientists and their institutions. But where do university researchers, with their traditional focus on publications and long-term projects, fit into a fast-moving ecosystem that supporters hope will also offer huge gains for public health?
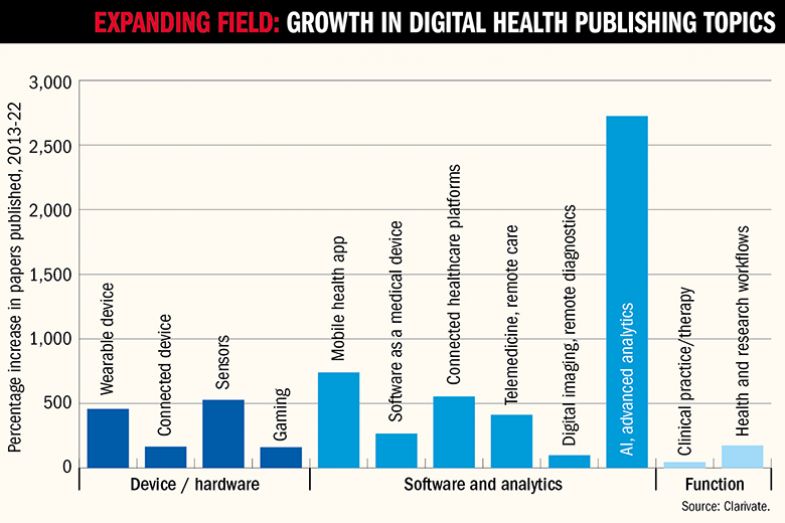
Given that the average cost of developing a new drug is about £1.8 billion, according to a 2023 Deloitte report, it is easy to see why a relatively low-cost technical solution might be appealing to healthcare funders, particularly governments keen to free up resources for other things. That focus on reducing costs is understandable but should not be the main driver of digital health research, says Amy Hardy, a clinical psychologist and lecturer at King’s College London and co-founder of SloMo, a digital therapy app that aims to help people minimise paranoid thoughts.
Supported by a £1.3 million Wellcome Trust grant, SloMo helps people to visualise their thoughts as fast- or slow-spinning “worry bubbles”, encouraging users to slow down the “fast thinking” often linked to episodes of psychosis. Randomised controlled trials have shown how SloMo, alongside in-person therapy, leads to significantly improved well-being levels compared with traditional counselling sessions.
“It’s a blended digital therapy, so it’s about adding to therapy, not reducing it,” explains Hardy. “I hope the direction of travel is increasing patient choice and personalising what’s on offer, rather than limiting choice to just digital.” Testing has shown that SloMo users may need fewer in-person sessions to reduce their sense of paranoia, which would be welcome news for the quarter of mental health patients in the UK who currently wait more than 12 weeks to start treatment.
Developed by Royal College of Art staff, design firm Special Projects and software firm BitJam in collaboration with King’s researchers, SloMo has been approved for use by several NHS trusts in the UK. But it could have a bigger future than as a clinical tool prescribed by psychiatrists, says Hardy, who notes that mental health and wellness apps can be big business. Most famously, the Sleepio digital sleep-improvement therapy, spun out of research conducted by University of Oxford neuroscientist Colin Espie, is used by millions of insomniacs, with its parent company, Big Health, already having attracted more than £100 million in funding to expand its mental health app services.
“This is a challenge for any academic in this field – you need some kind of commercialisation to scale up to meet the demand for an app, so you’ll have different agendas depending on your stakeholders,” reflects Hardy on balancing the clinical needs of patients with the desire to push a product into the mainstream.
“At the moment, our ideal outcome for SloMo is redundancy,” says Hardy. “We want people to stop using it [as their well-being improves]. That’s very different to the attention economy school of design used by some commercial companies.”
Others, however, are less nervous about heading down an unapologetically commercial road as long as research is supported. For Sarah Berry, chief scientist at ZOE, the British firm that has signed up more than 130,000 people to monitor their blood sugar, blood fat and gut health levels since its launch in 2022, the financial firepower of a venture capital-backed enterprise willing to invest in digital health research has delivered exciting research opportunities.
“Leveraging technologies allows me to advance from my previous randomised clinical trials [in nutrition studies], typically involving 20-30 people, to now run similar clinical trials with thousands of people, which is amazing,” says Berry, an associate professor of nutritional sciences at King’s – which is also the research base of Tim Spector, the twin studies expert whose popular Covid-era monitoring app has transformed into the PREDICT research programme, billed as “the largest in-depth nutritional research programme of its kind in the world”.
The nutrition trials Berry conducted at the start of her career in the early 2000s were, she reflects, “small-scale, with high precision and depth, but lacking in breadth and scale. We now know that we’re incredibly complex as individuals and, therefore, to unravel the complexity of our biology and how we live our lives, we need to collect data at scale.”
While this wasn’t previously possible, “thanks to digital technologies, wearable devices, remote clinical testing and community science, we can [now] collect data at scale, with precision, breadth and depth across a broad cross section of people,” Berry reflects. “This data allows us to disentangle the complex features that shape how an individual responds to food and deliver individualised advice with the greatest impact on health.”
ZOE has published some papers on relatively small cohorts, but much larger studies are in the pipeline, she says. “We’ve been brave and bold, leveraging exciting new developments in terms of novel technologies for population science and in terms of worldwide scientific collaborations. We also work across a whole breadth of health and lifestyle areas. This is quite unique as, typically in academic research, scientists – including myself previously – focus on very narrow areas that they become very specialist in and get a reputation for,” she says.
Now, thanks to the uptake of digital health technologies, “be it sleep, physical activity or nutrition, we can piece together the complexity of what shapes our health. At the heart of it, we believe that what we eat, but also who we are – our microbiome and our age, our sex and lots of other exposures – all have an impact,” says Berry. “So what we’re doing at ZOE...is to collect more anonymised data for research purposes at an unprecedented scale and breadth so that we can get a very high-resolution picture to understand what the key determinants of health are at an individual level and offer actionable and impactful advice…ultimately helping people make better food choices.”
The spectacular rise of ZOE – with its celebrity backers, such as television presenter Davina McCall and Dragons’ Den regular Steven Bartlett, branded Marks and Spencer kefir drinks, £81 million of venture capital and crowdfunded support – has led to some pushback, including against the scientists who so enthusiastically extol the products. Given the scale of the enterprise, is it harder for Berry to conduct rigorous research on nutrition – and report results that are potentially unhelpful for ZOE – given her involvement in what is now a major enterprise?
Absolutely not, she insists. “If our users’ health is improving, that’s how we know our product is working. So we do not want to, in any way, inflate any of our results and we don’t want to bias any of our results. Because if we implement weak science in our product then we are not going to see improvements in the health of our members. And that is what ultimately shows the success of our research and our product.”
In any case, she adds, “we don’t claim, unlike some other wellness companies, to have a silver bullet. We don’t claim to cure all with one magical ingredient. We acknowledge that improving health can be challenging, that it requires effort and that it requires knowledge – and that’s why scientific knowledge and research is at the heart of what we do.”
Far from worrying about any perceived conflicts of interest, Berry views the creation of a demonstrably useful product, backed by ongoing research, as a huge achievement. “We’re really proud that our research is not science for the sake of science. It’s research that we can actually implement in the product and that can actually have impactful results on people’s health. And it’s really important that our studies and our science contribute to health research in a way that can benefit public health too, not just our members.”
Top 25 institutions with citation impact
|
Rank |
Institution |
Digital health papers in the |
Citations |
Percentage of papers in the top |
CNCI |
|
1 |
Beth Israel Deaconess Medical Center |
70 |
1,444 |
28.57 |
4.11 |
|
2 |
51 |
532 |
17.65 |
3.49 |
|
|
3 |
50 |
1,011 |
26.00 |
2.59 |
|
|
4 |
75 |
1,582 |
32.00 |
2.50 |
|
|
5 |
284 |
4,885 |
28.52 |
2.46 |
|
|
6 |
World Health Organization |
53 |
917 |
28.30 |
2.22 |
|
7 |
Brigham & Women’s Hospital |
68 |
1,116 |
22.06 |
2.20 |
|
8 |
130 |
2,844 |
26.92 |
2.10 |
|
|
9 |
129 |
2,899 |
26.36 |
1.98 |
|
|
10 |
131 |
2,631 |
21.37 |
1.93 |
|
|
11 |
UCL |
198 |
4,021 |
30.81 |
1.92 |
|
12 |
60 |
746 |
26.67 |
1.85 |
|
|
13 |
Massachusetts General Hospital |
80 |
1,350 |
30.00 |
1.83 |
|
14 |
55 |
634 |
25.45 |
1.70 |
|
|
15 |
66 |
1,200 |
24.24 |
1.64 |
|
|
16 |
69 |
933 |
15.94 |
1.60 |
|
|
17 |
81 |
1,535 |
24.69 |
1.59 |
|
|
18 |
71 |
1,532 |
25.35 |
1.58 |
|
|
19 |
University of Queensland |
68 |
668 |
22.06 |
1.56 |
|
20 |
72 |
1,446 |
22.22 |
1.51 |
|
|
21 |
Nanyang Technological University |
55 |
1,082 |
16.36 |
1.47 |
|
=22 |
134 |
1,935 |
20.90 |
1.45 |
|
|
=22 |
London School of Hygiene & Tropical Medicine |
58 |
1,182 |
17.24 |
1.45 |
|
24 |
163 |
1,765 |
16.56 |
1.43 |
|
|
25 |
87 |
841 |
20.69 |
1.42 |
Source: Clarivate. Note: CNCI = category normalised citation impact. This is a measure of the relative rate of citation for an institution given papers’ age and field classification (older papers will be more cited than younger ones and different fields carry different average rates of citation).
The vast streams of medical data emitted from thousands of wearable devices or mobile phones would have once overwhelmed even the most data-savvy researcher. But thanks to artificial intelligence, researchers do not need to be skilled coders to meaningfully navigate such oceans of information. Similarly, hospitals and health centres across the world are sharing patient records in a way that lets algorithms pick up trends, even identifying new pandemics in their early stages.
But the lack of standardisation in operating systems is limiting the potential of the digital explosion to revolutionise public health, says Garrett Mehl, head of the digital health unit at World Health Organization. For instance, “one national health system might store data on cases of fever, but [the data] won’t say what fever [it is] or how it is characterised. If you had the same software operating with a common language, that would be a game-changer for public health,” says Mehl. With technology companies keen to “lock in” customers to their software, the world needs to agree a “common grammar” in health software to stop the “chaos we’ve seen for many years with all these different operating systems”, he says.
Calls to make more of the UK’s NHS data “AI-ready” were recently made in a joint report by former UK prime minister Tony Blair and former Conservative Party leader William Hague in their recent blueprint for promoting digital health innovations. But Mehl is clear that interoperability needs to work in both directions.

“The internet was built with open source technologies like Linus and Apache, which meant you didn’t have to rebuild the system to get something done,” he says. “We need the same for health so that an engineer in Kenya or a researcher in India will be able to work within a recognisable system when they’re doing analysis.”
It is also important that those in developing economies are not priced out of accessing potentially transformative digital medicine innovations, adds Mehl. “We’re starting to see how large language models trained on medical corpuses can be used to augment provision in rural areas,” he says. “This might help to advise a less skilled medical worker, who could refer a case, via telemedicine, to a specialist with the right training to make a diagnosis.”
Innovators who create such systems should be financially rewarded, Mehl agrees, but he is keen that groundbreaking interventions remain open to those who most need them. “The locking down of intellectual property has, from our perspective at the WHO, been a huge burden on middle- and low-income countries,” he says.
Interestingly, while the Clarivate analysis reveals that the top eight most prolific countries in terms of digital health publications produced between 2013 and 2022 are Western, universities from the developed world are not among the most prolific filers of digital health patents. US researchers were among the authors of 36 per cent of all papers, while UK researchers were involved in 20 per cent and Australia in 10 per cent. China ranked only ninth for involvement in publications, with its authors listed on 3.6 per cent of papers, yet the Chinese Academy of Science ranks alongside the likes of Samsung, Apple and Alphabet among the most prolific filers of digital health patents – and two Chinese universities filed more than Chinese tech firm Huawei.
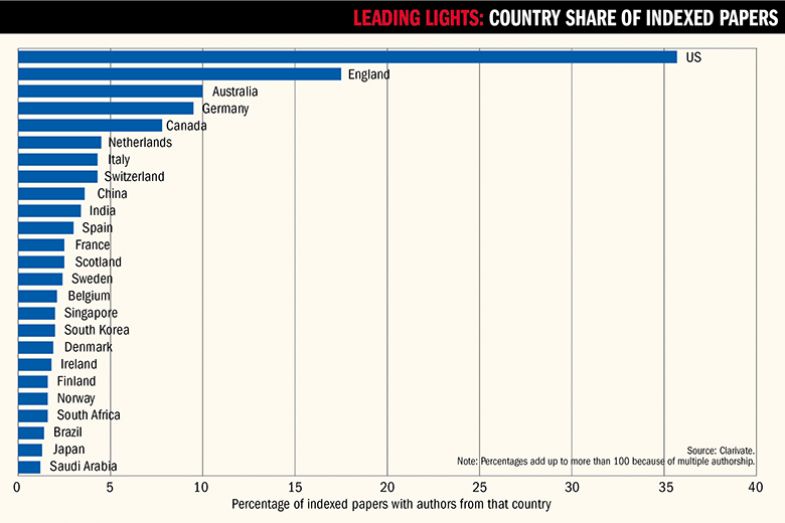
This perhaps suggests that Chinese universities are more focused on revenue generation from digital health than Western universities are, even though the vast country – alongside fellow Asian giant India, which ranks 10th for publishing output but is not a prolific patent-filer – potentially stands to gain massively if the cost savings promised by digital health are realised. (The analysis also highlights Japan’s surprisingly small involvement in publishing on digital health, being involved in just 1.3 per cent of papers despite its large elderly population and its highly developed tech sector.) According to Gaurav Agnihotri, an associate director at Clarivate, Chinese universities “get a subsidy from the state governments for filing patents, and even rewards in some cases when the patent gets granted. However, most of these universities and institutes have a narrow approach to patent protection as they rarely file for protection outside of China. Patent filings from corporates (and non-state-owned entities) are generally more strategic and more directed towards commercialisation.”
In richer countries, the pace of adoption of digital health by health systems is “not a resource question”, says Andrew Farmer, professor of general practice at Oxford, who, with the University of Warwick’s Dan Lasserson, is leading a large project to establish “virtual wards” in the homes of acutely ill patients, closely monitoring them via remote sensors.

“These interventions are not expensive if you compare them to drug costs or building a hospital...Authorities might just want to buy them anyway to see if they work. But medical technology should, I believe, have the same rigour of research applied to it as drugs or other interventions,” says Farmer, whose trial is funded by the UK’s National Institute for Health and Care Research (NIHR).
For Lasserson, professor of acute ambulatory care at Warwick, the big barrier to studying digital health interventions for high-need patients is not cost or patient consent but training staff to work differently. “I am training nurses and pharmacists to do things they’ve never done before, such as ultrasound imaging in people’s homes, which mean I don’t need to be there,” he says.
But providing such training on a large scale requires a level of “organisational slack” that the NHS does not currently enjoy, argues Trish Greenhalgh, professor of primary health medicine at the University of Oxford. “People forget to fund that bit: the time you need to teach people how to work differently. And no one has any time to spare at the moment,” she says, adding that the training and implementation side of health innovation also needs studying.
“When you’re using a new piece of technology, somebody – or an entire system – is having to do something differently, so the change is as much social as it is technological,” she says. That also goes for patients – which is why Greenhalgh thinks that studies of the impact of moving medical consultations online, as happened during the pandemic, should focus not just on the efficiency gains but also on who might have been excluded by the switch. “Those teleconferences worked for transactional exchanges for those with high health literacy, but it’s wrong for those with complex needs or those who might be in difficult [personal] relationships,” she says.
That view is backed up in several of the most highly cited papers published during the pandemic, according to the Clarivate analysis. “Poverty, lack of access to digital health, poor engagement with digital health for some communities, and barriers to digital health literacy are some factors that can contribute to poor health outcomes,” read one 2020 paper, “Digital health equity and COVID-19: the innovation curve cannot reinforce the social gradient of health”, published in the Journal of Medical Internet Research – the journal that has published the biggest share of digital health papers.
In the UK’s case, however, it is primarily the “torturous” system of NHS procurement that is holding back the adoption of useful digital health technologies, Greenhalgh says. Even when “some clever person has come up with an amazing piece of technology…the NHS cannot buy – or will not – because of red tape”, she explains.
That view is echoed by Roberto Liddi, who chairs the Association of British Healthtech Industries’ Digital Health Group. “It’s easier for a UK manufacturer to enter the US medical device market than the UK’s,” he explains. That is because each NHS trust will have “similar but slightly different” rules depending on geography or demographics, which means that sales pitches need to be tweaked for each one. “Sheffield has different rules to Manchester, which means you need a lot of sales power to crack that NHS market. One of my products got into 42 hospitals, but it required a gigantic effort and only really happened through word-of-mouth recommendations.
This means that while digital heath innovations are, in medical terms, fairly low cost, start-ups can nevertheless burn their capital quickly as they struggle to gain clinical approval before landing their first big customers, says Liddi. “The cost of the average digital health app is about £250,000 to £300,000 and the average concept-to-market period is 12 to 18 months,” he says. “That’s why you’ll see lots of ideas coming from universities, but rarely do they bring them forward themselves.”
With UK regulation covering the safety of devices, their effectiveness in clinical settings and their handling of patient data, it’s not easy to bring products to market, says Liddi. But given the non-invasive, light-touch nature of many digital therapies, there should be room to speed up the process, he believes. If a standard piece of NHS kit, such as a heart-rate monitor, malfunctions, “that could be a serious risk if you have a heart attack,” he says. “But that same risk doesn’t apply to an app that monitors your sleep apnoea and gives you advice – the worst that will happen is a bad night’s sleep.”

For Liddi, the clinical case for many of the devices that the UK produces is overwhelming. “It’s not a question of safety any longer – it’s more about the political will to level the entrance requirements so these things can be introduced at a national level,” he says.
For instance, many of the standard GP checks of blood pressure and other vital signs could be done via relatively low-cost devices at home. “If those vital signs were sent directly to your GP, he or she could monitor 10 or 20 patients at a time and see those who need to be seen,” Liddi says. “We’re talking efficiency gains of a [factor] of 10 or 20, and you could apply the same things to [emergency] waiting rooms.”
Moreover, the first country to really get approvals right could become a magnet for digital health entrepreneurs, Liddi says. But even in the US’ supposedly more entrepreneurial environment, “few strategies exist to help tools cross the chasm from clinical validation to integration within the workflows of a large health system”, and “many previously proposed frameworks for digital health implementation are difficult to operationalise in these dynamic organisations”, according to one of the most-cited digital health papers from 2022 identified by Clarivate: “Deploying digital health tools within large, complex health systems: key considerations for adoption and implementation”, published in Nature Digital Medicine.
Moreover, in the US health system, built around private insurance, Stanford’s Snyder sees other problems. “There’s the scepticism of the medical establishment towards change, but there is also no incentive for doctors or hospitals to keep you healthy,” he says, given that their business models depend on treating ill health.
On the other hand, the insurers who cover medical costs are likely to see things very differently. “Personally, I think your health insurer should pay you to wear a smartwatch or glucose monitor, particularly if you’re diabetic like me, as it would save them huge amounts of money,” Snyder says.
After all, even meticulous and experienced trackers of diet like himself never stop learning about what’s good for them.
“I love eating pulled pork, but I didn’t understand why it kept spiking my blood sugar,” he says. “Then someone told me, ‘Everyone knows they add sugar to it’. It sounds obvious, but it’s not unless you can measure these things.”
Register to continue
Why register?
- Registration is free and only takes a moment
- Once registered, you can read 3 articles a month
- Sign up for our newsletter
Subscribe
Or subscribe for unlimited access to:
- Unlimited access to news, views, insights & reviews
- Digital editions
- Digital access to THE’s university and college rankings analysis
Already registered or a current subscriber? Login









